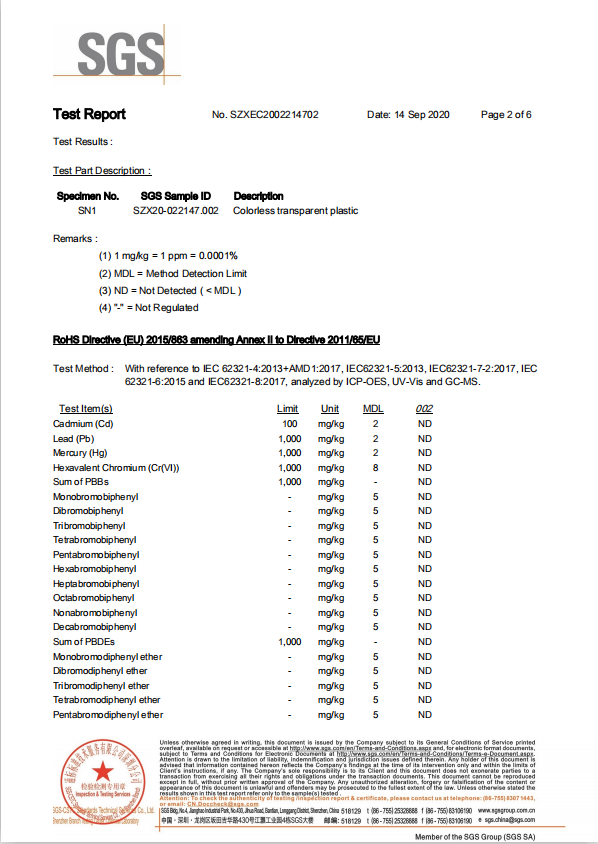
0092The Application of Silver PVC Film and Aluminum Foil in Pharmaceutical Blister Packaging
Material: PVC ROLL
Thickness 0.45-0.018mm-customization
Size: 80MM/130MM/Customization
GRADE: pharma grade film
color: white/silver customization
location: china
Usage: Pharmaceutical, dried food
- TOPLEADER
- 中国
- 15WORKING DAYS
- 5000T/M
- Information
- Video
- Download
The Application of Silver PVC Film and Aluminum Foil in Pharmaceutical Blister Packaging
I. Introduction to Pharmaceutical Blister Packaging
In the modern pharmaceutical industry, packaging plays a pivotal role in safeguarding the integrity and efficacy of drugs. Pharmaceutical blister packaging has emerged as one of the most prevalent and reliable forms of drug packaging. It not only provides a physical barrier against external contaminants such as moisture, light, and air but also offers convenience in dosing and storage.
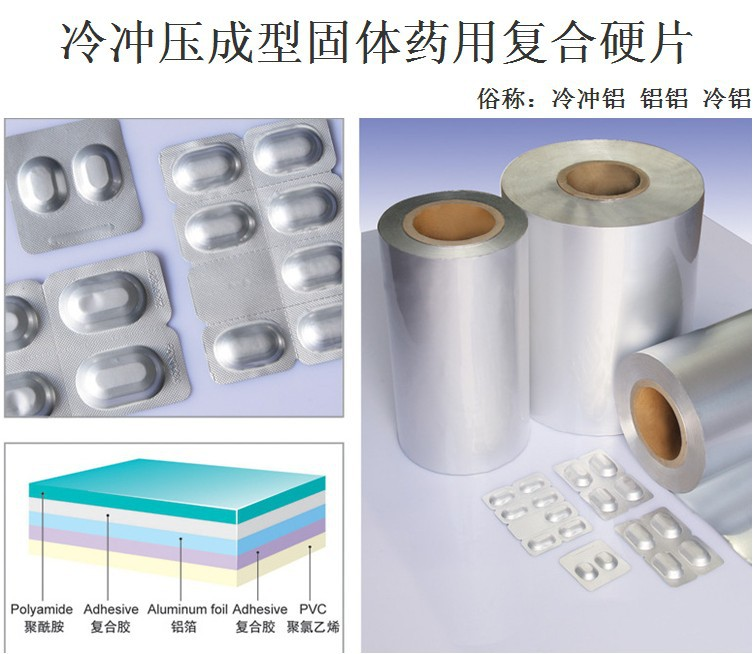
The two commonly used materials in pharmaceutical blister packaging are silver PVC film and aluminum foil, each possessing unique properties that contribute to the overall functionality and effectiveness of the packaging. This article delves into the characteristics, applications, advantages, and considerations associated with the use of silver PVC film and aluminum foil in pharmaceutical blister packaging.
II. Characteristics of Silver PVC Film
II.1. Material Composition and Basic Properties
Silver PVC film is primarily composed of polyvinyl chloride (PVC), a synthetic polymer made by polymerizing vinyl chloride monomers. The main chemical components include carbon, hydrogen, and chlorine atoms. PVC resins provide the basic framework, and various additives are incorporated during the manufacturing process. These additives can enhance specific properties such as flexibility, stability, and coloration.

Silver PVC film exhibits certain transparency, allowing for visual inspection of the packaged pharmaceuticals to some extent. It possesses good flexibility, enabling it to be easily formed into different shapes during the blister packaging process. This flexibility is crucial for accommodating various drug forms and sizes. In terms of durability, it can withstand normal handling and storage conditions without significant deformation or damage. However, its durability may be affected by factors such as temperature, humidity, and exposure to certain chemicals.
The transparency of silver PVC film is beneficial for quickly identifying the drug inside the package, which is convenient for both patients and healthcare providers. Its flexibility allows for efficient production of blister cavities with precise shapes and sizes, ensuring a snug fit for the drugs. The durability ensures that the packaging remains intact during transportation and storage, protecting the drugs from physical damage.
II.2. Advantages in Pharmaceutical Blister Packaging
One of the significant advantages of silver PVC film in pharmaceutical blister packaging is its excellent formability. It can be thermoformed into a wide variety of blister cavity designs to accommodate different drug forms, such as tablets, capsules, and lozenges. This versatility in shaping allows for efficient packaging of various medications, maximizing the use of available space and reducing waste.
Silver PVC film also offers relatively good resistance to impact. During handling, transportation, and even accidental drops, it can absorb and dissipate the energy, protecting the drugs from breakage or damage. This impact resistance is essential for maintaining the integrity of the drug products and ensuring their efficacy.
Cost-effectiveness is another appealing aspect of silver PVC film. Compared to some alternative packaging materials, it is more affordable, which is an important consideration for pharmaceutical manufacturers, especially when dealing with large production volumes. The lower cost without sacrificing basic packaging functions makes it a popular choice for many pharmaceutical products.
For example, in the packaging of common over-the-counter medications, the formability of silver PVC film allows for the creation of blisters that can hold different shapes and sizes of tablets. The impact resistance ensures that the tablets remain intact even if the package is mishandled during shipping or in a retail environment. The cost-effectiveness of using silver PVC film helps keep the overall production cost of these medications in check, making them more accessible to consumers.
II.3. Disadvantages and Limitations
Despite its advantages, silver PVC film has certain disadvantages and limitations. Under specific conditions, such as high temperatures or prolonged exposure to certain substances, it may release harmful substances. For instance, when heated above its recommended temperature range, it can potentially emit chlorine-based compounds, which could pose a risk to the quality and safety of the packaged drugs.

Its resistance to some chemicals is relatively poor. In a storage environment where there are strong acids, alkalis, or solvents, the PVC film may undergo chemical reactions that could compromise its integrity and barrier properties. During sterilization processes, if inappropriate sterilization methods are used, the film may also be affected, leading to a reduction in its performance.
The barrier properties of silver PVC film, particularly its ability to prevent the ingress of moisture and oxygen, are limited compared to some other packaging materials. Over time, this limited barrier performance may affect the long-term stability of certain drugs, especially those that are sensitive to moisture or oxidation. For example, drugs with hygroscopic properties may absorb moisture through the PVC film, leading to degradation or loss of potency.
In conclusion, while silver PVC film offers several benefits in pharmaceutical blister packaging, its limitations must also be carefully considered. Pharmaceutical manufacturers need to evaluate the specific requirements of each drug product and the overall packaging environment to determine whether silver PVC film is the most suitable choice or if additional measures need to be taken to mitigate its drawbacks.
III. Characteristics of Aluminum Foil
III.1. Material Features and Composition
Aluminum is a lightweight metal with a density of approximately 2.7 g/cm³. It has a face-centered cubic crystal structure, which endows it with good ductility and malleability. Pure aluminum is relatively soft, so in the production of aluminum foil for pharmaceutical packaging, certain alloying elements are often added to improve its strength and other properties. The most commonly used alloy is 8011 aluminum alloy, which contains elements such as iron and silicon. These alloying elements can enhance the hardness and tensile strength of the aluminum foil while maintaining its good formability.
The production process of aluminum foil involves multiple rolling steps. First, aluminum ingots are heated and rolled into thick sheets, and then through continuous cold rolling, the thickness is gradually reduced to the required level, usually ranging from 0.02 mm to 0.03 mm for pharmaceutical packaging. During this process, the surface of the aluminum foil becomes smooth, exhibiting a unique metallic luster. This smooth surface is not only aesthetically pleasing but also beneficial for subsequent printing and coating operations.
The purity of aluminum foil used in pharmaceuticals is generally high, usually above 99%. High purity ensures that the aluminum foil has good chemical stability and does not release harmful substances that could contaminate the drugs. The alloy composition is carefully controlled to meet the specific requirements of pharmaceutical packaging, such as ensuring appropriate mechanical strength and barrier properties.
III.2. Superiorities in Pharmaceutical Blister Packaging
One of the most outstanding features of aluminum foil is its excellent light-blocking ability. It can almost completely block ultraviolet, visible, and infrared light, effectively protecting light-sensitive drugs from degradation caused by light exposure. For example, many antibiotics, vitamins, and certain hormones are sensitive to light. When packaged with aluminum foil, the drug's chemical stability is significantly enhanced, maintaining its potency and quality over a longer period.
Aluminum foil also demonstrates outstanding barrier properties against gases such as oxygen and moisture. The extremely low permeability of aluminum foil to these gases prevents the ingress of oxygen and moisture, which are common factors contributing to drug degradation. This is crucial for drugs that are prone to oxidation or hydrolysis, such as some cardiovascular drugs and certain traditional Chinese medicine preparations. By maintaining a low-oxygen and low-moisture environment inside the package, the efficacy and shelf life of the drugs are prolonged.
In terms of hygiene and safety, aluminum foil meets strict pharmaceutical packaging standards. It is non-toxic, odorless, and does not interact chemically with the packaged drugs. This ensures that the drugs remain pure and uncontaminated during storage and transportation. The smooth surface of the aluminum foil also prevents the adhesion of dust and microorganisms, further enhancing the hygienic quality of the packaging.
III.3. Drawbacks and Considerations
Compared to some plastic packaging materials, the cost of aluminum foil is relatively high. The production process of aluminum foil, including the extraction and refining of aluminum ore, as well as the complex rolling and processing procedures, contributes to its higher cost. This may pose a challenge for pharmaceutical manufacturers, especially when dealing with large-scale production of low-cost generic drugs. In such cases, alternative packaging materials with lower costs may be considered, although they may not offer the same level of performance as aluminum foil.
Aluminum foil has a certain degree of brittleness, especially when the thickness is very thin. During the packaging process, improper handling, such as excessive bending or stretching, can cause the aluminum foil to crack or break. This requires careful operation and the use of appropriate packaging machinery to ensure the integrity of the aluminum foil during the production of blister packs. Additionally, the sharp edges of the aluminum foil after cutting need to be carefully treated to prevent potential damage to the drugs or the packaging.
Regarding recyclability, aluminum foil is recyclable, but the recycling process is more complex compared to some other materials. It requires specialized recycling facilities and processes to separate and purify the aluminum foil from other packaging components. In some regions, the availability of such recycling facilities may be limited, which could affect the overall environmental friendliness of using aluminum foil. However, efforts are being made to improve the recycling efficiency and infrastructure to address this issue.
IV. The Blister Packaging Process Using Silver PVC Film
IV.1. Preparation of PVC Film
The sourcing of PVC film for pharmaceutical blister packaging is a crucial step. Reputable suppliers are selected to ensure the material meets the required quality standards. Upon receipt, the PVC film is inspected for various parameters. Visual inspection is carried out to check for any scratches, holes, or irregularities on the surface. Thickness measurements are taken at multiple points using precision instruments to ensure uniformity. This is important as inconsistent thickness can lead to uneven blister formation and affect the overall quality of the packaging.
The PVC film is typically stored in a controlled environment to prevent exposure to excessive heat, humidity, and sunlight, which could potentially degrade its properties. Before use, it may be conditioned to the appropriate temperature and humidity levels to enhance its formability during the blister forming process.
For example, in a pharmaceutical packaging facility, the PVC film is received in rolls and is first visually examined under proper lighting conditions. Any visible defects are marked and the affected areas are either trimmed or the entire roll is rejected if the defects are extensive. The thickness is measured using a micrometer at regular intervals across the width and length of the roll to ensure it falls within the specified tolerance range.
IV.2. Blister Forming Techniques
There are several methods for forming blisters from PVC film, with thermoforming and vacuum forming being the most commonly used. In thermoforming, the PVC film is heated to a temperature that makes it pliable. This temperature range is carefully controlled, usually between 100°C and 150°C, depending on the specific formulation of the PVC film. Once heated, the film is then molded into the desired blister shape using a male or female mold. Pressure is applied to ensure the film conforms precisely to the mold cavity.
Vacuum forming, on the other hand, involves heating the PVC film and then using a vacuum to draw the softened film into the mold. This process is particularly useful for creating complex shapes. The vacuum pressure is typically maintained at around 0.8 to 1.2 bar.
During these processes, parameters such as temperature, pressure, and time need to be precisely controlled. If the temperature is too high, the PVC film may become overheated and lose its mechanical properties, leading to weak or deformed blisters. If the pressure is too low or the time is insufficient, the film may not form properly, resulting in incomplete or misshapen blisters.
Modern machinery has significantly improved the precision and efficiency of blister forming with PVC film. Computer-controlled thermoforming and vacuum forming machines are now available, which can accurately regulate the process parameters. These machines are also equipped with advanced sensors and feedback systems to monitor and adjust the process in real-time, ensuring consistent quality and high production rates. For instance, a state-of-the-art thermoforming machine can produce hundreds of blisters per minute with minimal variation in size and shape.
IV.3. Sealing and Finishing Steps
After the blisters are formed, they need to be sealed to enclose the pharmaceuticals. Heat sealing is a commonly used method. The temperature for heat sealing is typically set between 120°C and 180°C, depending on the thickness and composition of the PVC film. The sealing time and pressure are also adjusted to ensure a proper seal. Another option is the use of adhesives, which are carefully selected to be compatible with the PVC film and provide a strong and reliable bond.
Quality checks are carried out to ensure the integrity of the seals. Visual inspection is done to check for any gaps or weak points in the seals. Leak tests may also be performed, where the sealed blisters are placed in a vacuum chamber or immersed in a liquid to detect any leakage.
Finishing touches include trimming the excess PVC film around the blisters to give a neat and clean appearance. Labeling is then carried out, providing essential information such as the drug name, dosage, expiration date, and batch number. The labels are applied using automated labeling machines to ensure accuracy and consistency.
In a pharmaceutical packaging line, the sealed blisters pass through a series of quality control stations. At the trimming station, sharp blades are used to precisely cut away the excess film. The labeled blisters are then packaged in cartons or other secondary packaging materials, ready for distribution to pharmacies and healthcare facilities.
V. The Blister Packaging Process Using Aluminum Foil
V.1. Preparation of Aluminum Foil
Aluminum foil used in pharmaceutical packaging is typically manufactured through a series of rolling processes. High-purity aluminum ingots are first heated and then passed through multiple sets of rollers to gradually reduce the thickness to the required level. The choice of aluminum alloy, such as 8011 alloy, is crucial as it imparts the necessary strength and formability. The foil is then annealed to improve its ductility and reduce internal stresses.
Surface treatment is often necessary to enhance the performance of aluminum foil. One common treatment is coating with a protective layer, such as an organic polymer or a lacquer. This coating serves multiple purposes. It protects the aluminum foil from oxidation, which could otherwise lead to the formation of aluminum oxide and compromise the foil's barrier properties. It also improves the adhesion of subsequent coatings or adhesives, ensuring a strong bond. For example, a thin layer of acrylic coating can be applied to increase the surface energy of the foil, facilitating better wetting and adhesion of inks during the printing process. Additionally, the coating can provide a smooth and even surface, enhancing the overall appearance of the printed and packaged product.
V.2. Blister Packaging Operations
The first step in incorporating aluminum foil into the blister packaging system is often printing. This can be done using various printing techniques such as gravure printing or flexographic printing. The printed information includes essential details such as the drug name, dosage, batch number, and expiration date. High-quality inks that are resistant to abrasion and chemical degradation are used to ensure the legibility and durability of the printed text and graphics.
After printing, a protective layer or adhesive may be coated onto the aluminum foil. If a protective layer is applied, it further safeguards the foil from scratches and other physical damage during handling and storage. When an adhesive is used, it is carefully selected to ensure compatibility with both the aluminum foil and the plastic sheet or tray with which it will be combined. The adhesive layer needs to provide a strong and reliable bond to maintain the integrity of the blister package.
The aluminum foil is then combined with other components, typically a plastic sheet or tray. The plastic sheet, often made of PVC or a similar material, is thermoformed into the blister cavities that will hold the pharmaceuticals. The aluminum foil is then precisely positioned and heat-sealed or laminated onto the plastic sheet. The heat-sealing process requires precise control of temperature, pressure, and time to ensure a proper seal. The sealed blister package provides a protective enclosure for the drugs, shielding them from environmental factors such as moisture, light, and air.
V.3. Quality Control and Inspection
For aluminum foil-based blister packages, several quality parameters are closely monitored. The integrity of the foil layer is of utmost importance. This includes checking for the presence of pinholes, which could allow the ingress of air and moisture, potentially compromising the drug's quality. Advanced inspection techniques such as pinhole detectors, which use optical or electrical methods to identify even the smallest pinholes, are employed. The adhesion between the aluminum foil and other layers, such as the plastic sheet or printed coatings, is also tested to ensure that there is no delamination or separation during the product's shelf life.
Visual inspection is carried out to check for any surface defects, scratches, or unevenness on the aluminum foil. The printed information is verified for accuracy, clarity, and durability. Additionally, the overall dimensions and shape of the blister package are measured to ensure they meet the specified tolerances. This is crucial as improper dimensions could affect the fit and protection of the drugs inside the package.
Advanced inspection techniques like machine vision systems are increasingly being used. These systems can quickly and accurately detect defects and deviations from the required quality standards. They can analyze the surface of the aluminum foil for any irregularities, check the alignment and quality of the printed text and graphics, and even measure the thickness and uniformity of the foil and other layers. By ensuring the highest quality of the final packaged products, pharmaceutical manufacturers can enhance patient safety and the effectiveness of the drugs they produce.
VI. Comparison of Their Performance in Protecting Drugs
VI.1. Shelf Life Extension
In terms of maintaining the chemical stability and potency of drugs over time, aluminum foil generally outperforms silver PVC film. Aluminum foil's excellent barrier properties against moisture, oxygen, and other gases significantly reduce the rate of drug degradation. For example, many solid oral medications, such as certain antibiotics and cardiovascular drugs, are sensitive to moisture and oxidation. When packaged with aluminum foil, the ingress of these detrimental factors is severely restricted, thereby prolonging the drug's shelf life. In contrast, silver PVC film, while providing some degree of protection, allows a relatively higher permeation rate of gases and moisture. Over an extended period, drugs packaged in silver PVC film may experience a more pronounced decline in potency and quality compared to those in aluminum foil packaging. However, for drugs with a relatively short shelf life or those that are less sensitive to environmental factors, the difference in shelf life extension between the two materials may be less significant.
VI.2. Protection Against External Factors
Aluminum foil's ability to shield drugs from light, moisture, oxygen, and other environmental factors is highly effective. Its light-blocking property is nearly complete, safeguarding light-sensitive drugs from degradation. In a real-world scenario, consider a pharmaceutical product that contains a photosensitive ingredient. When packaged in aluminum foil, the drug remains stable even under prolonged exposure to light, whereas if packaged in silver PVC film, which has some degree of transparency, the drug may be more susceptible to light-induced degradation. In terms of moisture and oxygen protection, aluminum foil's low permeability ensures that the drug is stored in a relatively dry and low-oxygen environment, preventing issues such as hydrolysis and oxidation. Silver PVC film, although it can offer some protection, is not as efficient as aluminum foil in this regard. In humid or oxygen-rich storage conditions, drugs packaged in silver PVC film may be at a higher risk of quality deterioration.
VI.3. Impact on Drug Quality and Efficacy
The use of aluminum foil in drug packaging has a positive impact on the overall quality and efficacy of the drugs when consumed by patients. The excellent barrier properties maintain the drug's chemical integrity, ensuring that the intended therapeutic effect is achieved. Additionally, the smooth surface of aluminum foil reduces the likelihood of drug adhesion and loss during the packaging and dispensing process. In contrast, silver PVC film's relatively inferior barrier properties may allow for some degree of drug degradation, which could potentially affect the dissolution rate and bioavailability of the drug. If a drug has been partially degraded due to inadequate protection by the packaging, its dissolution in the body may be slower or incomplete, leading to a reduced bioavailability and potentially compromising the treatment outcome. Therefore, for drugs where precise dosing and optimal efficacy are critical, aluminum foil is often the preferred choice to ensure the highest quality and effectiveness of the medication.
VII. Cost Analysis in Pharmaceutical Packaging
VII.1. Initial Material Costs
The purchase prices of silver PVC film and aluminum foil for pharmaceutical packaging applications can vary significantly. Generally, silver PVC film is more cost-effective in terms of initial material cost. The market price of silver PVC film typically ranges from Y per square meter, depending on factors such as thickness, quality grade, and supplier. For example, a common grade of silver PVC film with a thickness suitable for most pharmaceutical blister packaging may cost around $Z per square meter in bulk purchases.
On the other hand, aluminum foil is relatively more expensive. The price of aluminum foil for pharmaceutical use can range from B per square meter. The higher cost of aluminum foil is attributed to the more complex production process, including the extraction and refining of aluminum ore, as well as the need for precise rolling and processing to achieve the required thickness and quality.
The quantity of materials purchased can have a substantial impact on the unit cost. Larger orders often result in more favorable pricing due to economies of scale. Pharmaceutical manufacturers ordering large volumes of silver PVC film or aluminum foil may be able to negotiate lower prices per unit with suppliers. Quality grades also play a crucial role. Higher quality grades of both materials, which offer better barrier properties, durability, and printability, usually come at a premium price. For instance, a pharmaceutical company requiring a silver PVC film with enhanced barrier performance against moisture and oxygen may need to pay a slightly higher price compared to a standard grade.

Supplier agreements are another important factor. Long-term contracts with reliable suppliers can provide more stable pricing and potentially better terms, such as discounts, favorable payment conditions, and priority in supply during periods of high demand or material shortages.
VII.2. Processing and Production Costs
During the blister packaging process, the costs associated with processing silver PVC film and aluminum foil differ. For silver PVC film, the energy consumption in thermoforming or vacuum forming processes is relatively moderate. The machinery used for processing PVC film is generally less expensive compared to that for aluminum foil. However, the labor cost can be a significant factor, especially if the production process requires precise manual handling or frequent adjustments to ensure the quality of the blister formation. The wear and tear of the machinery used for PVC film processing are also considerations, as the molds and forming equipment may need to be replaced or maintained more frequently, depending on the production volume and the complexity of the blister designs.
In contrast, processing aluminum foil involves higher energy consumption, particularly in the rolling and annealing processes during its production. The machinery required for handling aluminum foil, such as high-precision printing and laminating equipment, is more costly. The labor cost for aluminum foil processing is relatively high as well, due to the need for skilled operators to manage the more complex and delicate production steps. Additionally, the brittleness of aluminum foil can lead to a higher rate of material waste if not handled properly, further increasing the overall production cost.
The complexity of the packaging process for each material has a direct impact on the overall production costs. For example, if a pharmaceutical product requires a custom-designed blister pack with intricate shapes and multiple compartments, the processing of both silver PVC film and aluminum foil will be more challenging and costly. However, aluminum foil may present additional difficulties due to its brittleness and the need for more precise control during lamination and sealing processes. In contrast, simpler blister pack designs with standard shapes and sizes can be produced more efficiently and at a lower cost for both materials, although the cost advantage of silver PVC film in such cases may be more pronounced.

VII.3. Long-Term Cost Considerations
Long-term costs related to packaging failures and recalls are significant considerations for pharmaceutical companies. If the packaging fails to protect the drugs effectively, it can lead to product recalls, which can be extremely costly in terms of lost revenue, damage to the company's reputation, and potential legal liabilities. Silver PVC film, with its relatively inferior barrier properties compared to aluminum foil, may pose a higher risk of packaging failures for drugs that are highly sensitive to moisture, oxygen, or light. For example, if a drug packaged in silver PVC film deteriorates due to excessive moisture ingress over time, it may result in a recall, incurring costs such as the disposal of the defective products, re-packaging, and compensation to customers.
Storage requirements also contribute to the long-term cost. Aluminum foil, with its excellent barrier properties, can provide a more stable storage environment for drugs, reducing the need for specialized storage conditions such as controlled humidity and temperature. This can result in lower energy costs for maintaining the storage facilities. In contrast, drugs packaged in silver PVC film may require more stringent storage conditions to ensure their quality and potency, which can increase the long-term operational costs.
Over time, the potential loss in drug quality due to inadequate packaging can have economic implications. If a drug's efficacy is compromised due to degradation caused by the packaging material, it may lead to ineffective treatment for patients. This could result in additional costs for the healthcare system, such as repeated treatments or the need for alternative medications. From the pharmaceutical company's perspective, it could lead to a loss of market share and customer trust.

In conclusion, while silver PVC film may offer lower initial material and processing costs for some pharmaceutical packaging applications, the long-term costs associated with potential product recalls, storage requirements, and drug quality degradation need to be carefully evaluated. Aluminum foil, despite its higher initial cost, may provide better cost-effectiveness in the long run, especially for drugs that are sensitive to environmental factors and where maintaining the highest quality and efficacy is crucial. Pharmaceutical manufacturers need to conduct a comprehensive cost-benefit analysis, taking into account both short-term and long-term costs, to determine the most suitable packaging material for each drug product.
VIII. Environmental Impact
VIII.1. Recyclability and Disposal
In the context of pharmaceutical packaging waste, the recyclability of silver PVC film and aluminum foil presents different challenges and opportunities. Silver PVC film, being a plastic material, is technically recyclable. However, in practice, its recycling rate is relatively low. The main reason is that during the recycling process, the PVC film needs to be separated from other packaging components, such as adhesives and printed layers, which is a complex and costly process. Additionally, the presence of additives in the PVC film may affect its quality and usability in the recycled form. In some regions, the recycling infrastructure for PVC is not well-developed, leading to a significant portion of silver PVC film from pharmaceutical packaging ending up in landfills or being incinerated.

Aluminum foil, on the other hand, is highly recyclable. It can be melted down and reused to produce new aluminum products. However, similar to silver PVC film, the recycling of aluminum foil from pharmaceutical packaging requires proper separation from other materials. The small size and often laminated nature of aluminum foil in pharmaceutical blister packs make the separation process more difficult. In some cases, the adhesive used to bond the aluminum foil to other layers may contaminate the recycled aluminum, reducing its quality. Despite these challenges, the recycling of aluminum foil is more economically viable compared to silver PVC film, as the value of aluminum as a raw material is relatively high.

Proper disposal methods for each material are essential to minimize their environmental impact. For silver PVC film, if it cannot be recycled, incineration should be carried out in facilities with proper air pollution control systems to reduce the emission of harmful substances such as dioxins and hydrochloric acid. Landfilling is also an option, but it should be done in a controlled landfill site to prevent the leaching of additives and other contaminants into the soil and groundwater.
For aluminum foil, recycling is the preferred disposal method. Many recycling centers and industries have established processes to handle and recycle aluminum foil. In addition to traditional recycling methods, some innovative approaches are being explored, such as the development of new separation technologies to improve the efficiency and purity of recycled aluminum.
VIII.2. Environmental Footprint During Production
The manufacturing of silver PVC film and aluminum foil has a significant environmental impact. The production of silver PVC film involves the polymerization of vinyl chloride monomers, which is an energy-intensive process. The extraction and production of the raw materials, such as chlorine and ethylene, also contribute to its environmental footprint. During the manufacturing process, emissions of volatile organic compounds (VOCs), greenhouse gases, and other pollutants are generated. The use of additives in the PVC film may also pose risks to the environment if not properly managed.
In terms of resource consumption, the production of silver PVC film requires significant amounts of water and energy. The production of aluminum foil also has a notable environmental impact. The extraction of aluminum ore through the Bayer process and the subsequent refining and rolling steps consume large amounts of energy. The electrolysis process used to produce aluminum is particularly energy-intensive, contributing to a significant portion of the carbon footprint. Additionally, the mining and refining of aluminum ore can result in the generation of waste materials, such as red mud, which requires proper disposal to prevent environmental contamination.
Comparing the environmental footprints of the two materials, aluminum foil generally has a higher energy consumption and carbon footprint during production due to the energy-intensive nature of aluminum extraction and refining. However, silver PVC film has its own set of environmental concerns related to the use of chlorine and the potential release of harmful substances. To reduce these impacts, several measures can be taken. For silver PVC film, the development and use of more environmentally friendly additives and production processes, such as the reduction of chlorine content and the implementation of cleaner production technologies, can help minimize its environmental footprint. For aluminum foil, efforts can be made to improve the energy efficiency of the production process, such as the adoption of advanced electrolysis technologies and the use of recycled aluminum as a raw material to reduce the need for primary aluminum production.

VIII.3. Sustainable Alternatives and Future Trends
In recent years, there has been a growing interest in developing and using sustainable packaging materials in the pharmaceutical industry. Several emerging materials show potential to replace or be used in combination with silver PVC film and aluminum foil. One such material is biodegradable polymers, such as polylactic acid (PLA) and polyhydroxyalkanoates (PHA). These materials offer the advantage of being biodegradable, reducing the long-term environmental impact of packaging waste. However, they also have limitations in terms of barrier properties and cost, which need to be further improved to be widely applicable in pharmaceutical blister packaging.
Another alternative is the use of paper-based materials with appropriate coatings to enhance their barrier properties. These materials are renewable and have a lower environmental impact compared to plastics and metals. However, their performance in protecting drugs from moisture, oxygen, and light may not be as good as aluminum foil, and further research and development are needed to optimize their properties.
The future trends in the pharmaceutical packaging industry are likely to focus on more environmentally friendly options. Pharmaceutical companies are increasingly aware of the importance of sustainable packaging and are taking steps to reduce their environmental footprint. This includes the use of thinner and more efficient packaging materials to reduce material consumption, the development of recyclable and biodegradable packaging solutions, and the implementation of more sustainable production processes.

In addition, there is a growing trend towards the use of intelligent packaging technologies that can provide real-time information about the quality and integrity of the packaged drugs. These technologies can help reduce waste by ensuring that only high-quality drugs are distributed and used, and can also improve patient safety by providing alerts in case of packaging failures or drug degradation.
Overall, the future of pharmaceutical packaging will likely see a combination of new materials, improved production processes, and advanced technologies to meet the dual demands of protecting drugs and minimizing environmental impact.
IX. Regulatory Requirements and Standards
IX.1. International Standards for Pharmaceutical Packaging
In the global pharmaceutical industry, there are several major international regulations and standards that govern the use of packaging materials like silver PVC film and aluminum foil. One of the most prominent is the International Organization for Standardization (ISO) standards. For example, ISO 11607 specifies the requirements for packaging materials and systems intended to maintain the sterility of medical devices and pharmaceuticals. It outlines criteria for materials' barrier properties, seal integrity, and resistance to environmental factors such as temperature and humidity.
The United States Pharmacopeia (USP) also sets strict standards for pharmaceutical packaging materials. It includes tests for extractables and leachables from packaging materials to ensure that no harmful substances are transferred to the drugs. In the European Union, the European Pharmacopoeia has similar regulations, with a focus on ensuring the safety and quality of packaged drugs. These standards are designed to prevent contamination, maintain drug stability, and protect patients from potential harm caused by packaging materials.
Compliance with these international standards is crucial for pharmaceutical companies that export their products or operate in multiple regions. It ensures that the packaging materials used, including silver PVC film and aluminum foil, meet the required safety and quality benchmarks, thereby safeguarding the integrity of the drugs and the health of consumers.

IX.2. Specific Requirements for PVC and Aluminum Foil
Regarding silver PVC film, there are specific requirements and restrictions. In terms of chemical composition, the amount of residual vinyl chloride monomer (VCM) is strictly limited. For example, the European Union has set a maximum limit of 1 ppm (parts per million) for VCM in PVC used for food and pharmaceutical packaging. This is because VCM is a known carcinogen, and even trace amounts can pose a risk to human health. The physical properties such as tensile strength, elongation at break, and tear resistance are also specified to ensure that the film can withstand the rigors of the packaging process and protect the drugs during handling and storage.
For aluminum foil, the purity of the aluminum is typically required to be above 99%. The alloying elements, if present, must be carefully controlled to ensure that they do not affect the foil's performance and safety. The thickness of the foil used in pharmaceutical packaging is usually within a specific range, such as 0.02 mm to 0.03 mm, to provide the necessary barrier properties. Additionally, the surface quality of the aluminum foil is important, with requirements for smoothness and absence of scratches or pinholes.
Manufacturers need to comply with these specific requirements to enter the pharmaceutical packaging market. This involves rigorous testing and quality control measures. For example, they must conduct tests using advanced analytical techniques such as gas chromatography to measure the residual VCM in PVC film. For aluminum foil, they need to use precision instruments like thickness gauges and pinhole detectors to ensure that the foil meets the specified thickness and quality standards.

IX.3. Quality Assurance and Compliance Monitoring
Regulatory bodies and industry organizations have established mechanisms for quality assurance and compliance monitoring of pharmaceutical packaging materials. The Food and Drug Administration (FDA) in the United States conducts inspections and audits of pharmaceutical manufacturing facilities, including the packaging operations. They review the documentation of quality control tests, the sourcing of materials, and the manufacturing processes to ensure compliance with the regulations.
Industry organizations also play a role in promoting quality assurance. For example, the International Society for Pharmaceutical Engineering (ISPE) provides guidelines and best practices for pharmaceutical packaging. They conduct training programs and workshops to educate manufacturers about the latest standards and techniques for ensuring the quality of packaging materials.
To ensure that silver PVC film and aluminum foil used in packaging meet the required standards, manufacturers implement a series of quality control steps. This includes incoming material inspection, where samples of each batch of PVC film and aluminum foil are tested for various parameters such as thickness, barrier properties, and chemical composition. During the production process, in-process controls are carried out to monitor the blister forming, sealing, and printing operations. Finished product inspection involves testing the final packaged products for integrity, label accuracy, and compliance with the regulatory requirements.
In case of non-compliance, regulatory actions can be taken. This may include product recalls, fines, and restrictions on the manufacturer's operations. Therefore, it is essential for pharmaceutical companies and packaging material suppliers to maintain strict quality control and compliance with the regulations to ensure the safety and effectiveness of the drugs they package.

X. Case Studies of Pharmaceutical Products Using These Materials
X.1. Examples of Products Packaged with Silver PVC Film
Over-the-Counter Analgesics: Many common pain relievers, such as aspirin and acetaminophen tablets, are often packaged using silver PVC film. The formability of the PVC film allows for the creation of blisters that can neatly hold the tablets. The relatively low cost of silver PVC film is beneficial for mass-produced over-the-counter medications, helping to keep the retail price affordable for consumers. In terms of performance, the impact resistance of the PVC film protects the tablets during handling and transportation. For example, during the shipping process, the tablets are less likely to break or chip. Customer feedback has been generally positive regarding the ease of use. The transparency of the film enables users to quickly identify the tablets inside, and the blister packaging is convenient for dosing, allowing users to easily pop out the required number of tablets.
2. Vitamin Supplements: Some vitamin tablets and capsules are also packaged with silver PVC film. The flexibility of the PVC film is advantageous for accommodating different shapes and sizes of vitamin supplements. The ability to thermoform the film into various blister cavity designs ensures a snug fit for the products. From a performance perspective, it provides a certain level of protection against moisture and air, although not as effective as aluminum foil. For instance, in a normal household storage environment, the PVC film can prevent excessive moisture ingress for a period, maintaining the quality of the vitamins. Customers appreciate the clear visibility of the products and the convenience of the blister packaging, which makes it easy to organize and store the vitamins.
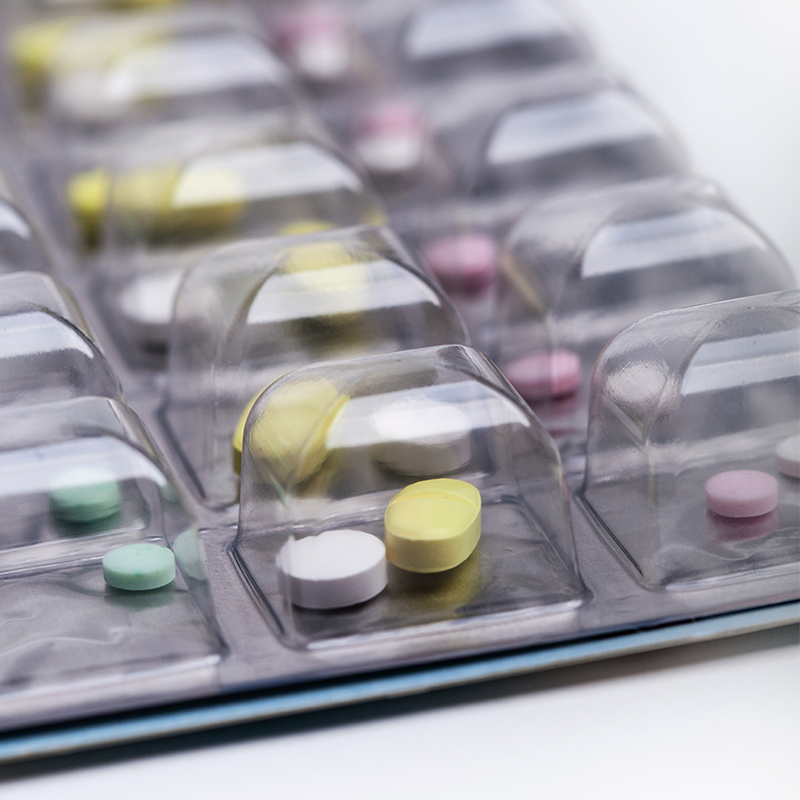
X.2. Examples of Products Packaged with Aluminum Foil
Antibiotics: Many antibiotics, especially those that are sensitive to light and moisture, are packaged with aluminum foil. For example, certain cephalosporin antibiotics require a high level of protection to maintain their potency. The excellent light-blocking and barrier properties of aluminum foil prevent the degradation of the antibiotics caused by exposure to light and the ingress of moisture and oxygen. The use of aluminum foil has significantly improved the stability of these drugs during storage and transportation. However, during the packaging process, special care needs to be taken due to the brittleness of the aluminum foil. Pharmaceutical manufacturers need to ensure precise handling and use appropriate machinery to avoid foil cracking or breakage. In some cases, the sharp edges of the aluminum foil after cutting may also pose a challenge, and measures need to be taken to prevent potential damage to the drugs or the packaging.
2. Hormone Medications: Hormone drugs, such as thyroid hormones and some steroid hormones, are often packaged with aluminum foil. These drugs are highly sensitive to environmental factors, and aluminum foil provides the necessary protection to ensure their efficacy. The smooth surface of the aluminum foil also helps prevent the adhesion of the drugs, reducing the risk of drug loss during the packaging and dispensing process. The strict quality control requirements for these medications necessitate the use of aluminum foil to maintain the highest quality and stability. However, the higher cost of aluminum foil compared to some other materials is a factor that pharmaceutical companies need to consider. They need to balance the cost with the importance of protecting the integrity and efficacy of the hormone medications.

X.3. Comparative Analysis of Case Studies
Packaging Performance: In the case of products packaged with silver PVC film, such as over-the-counter analgesics and vitamin supplements, the packaging provides a certain degree of protection against physical damage and some limited protection against environmental factors. However, compared to aluminum foil, the barrier properties are inferior. For drugs packaged with aluminum foil, like antibiotics and hormone medications, the excellent light-blocking, moisture-proof, and oxygen-barrier properties ensure a much higher level of protection, significantly reducing the risk of drug degradation. This difference in packaging performance is crucial for drugs with different sensitivities to environmental factors. For highly sensitive drugs, the use of aluminum foil is essential to maintain their quality and efficacy.
2. Market Acceptance: Over-the-counter products packaged with silver PVC film are widely accepted in the market due to their cost-effectiveness and convenience. The transparency of the film and the ease of use contribute to their popularity among consumers. For products packaged with aluminum foil, such as certain prescription medications, market acceptance is based on the reputation of the drug and the importance of protecting its potency. Healthcare providers and patients are more likely to trust the quality and stability of drugs packaged with aluminum foil, especially for those medications where any degradation could have significant consequences. However, the higher cost of aluminum foil may limit its use in some cost-sensitive applications.
3. Lessons Learned: From these case studies, it is evident that the choice of packaging material should be based on a careful consideration of the drug's characteristics, its sensitivity to environmental factors, and the overall cost-benefit analysis. For drugs that are less sensitive and cost is a major factor, silver PVC film can be a viable option, provided that appropriate measures are taken to mitigate its limitations. For drugs that require a high level of protection, aluminum foil is the preferred choice, despite the higher cost. Additionally, continuous improvement in the packaging process and quality control is essential for both materials to ensure the integrity and safety of the packaged drugs. Pharmaceutical companies should also stay updated with the latest advancements in packaging materials and technologies to optimize their packaging strategies and meet the evolving needs of the market and regulatory requirements.

XI. Conclusion
In conclusion, both silver PVC film and aluminum foil play significant roles in pharmaceutical blister packaging, each with its own set of characteristics, advantages, and limitations. Silver PVC film offers good formability, impact resistance, and cost-effectiveness, making it suitable for a wide range of pharmaceutical products, especially those that are less sensitive to environmental factors and where cost is a major consideration. However, its potential release of harmful substances under certain conditions and relatively poor barrier properties compared to aluminum foil must be carefully evaluated.
On the other hand, aluminum foil provides excellent light-blocking, barrier properties against moisture and oxygen, and high hygienic and safety standards. It is the preferred choice for drugs that are highly sensitive to light, moisture, and oxidation, ensuring the long-term stability and efficacy of the medications. Nevertheless, its higher cost and certain handling difficulties due to brittleness need to be taken into account.
For the future development of pharmaceutical packaging materials, there is a growing trend towards more sustainable and environmentally friendly options. Research and development efforts are focused on improving the recyclability and reducing the environmental footprint of packaging materials. At the same time, the pursuit of better barrier properties, cost-effectiveness, and compatibility with drugs will continue. Pharmaceutical companies need to conduct comprehensive evaluations, considering factors such as drug characteristics, regulatory requirements, cost-benefit analysis, and environmental impact, to select the most appropriate packaging material for each specific drug product. This will ensure the quality, safety, and effectiveness of pharmaceuticals while also meeting the demands of sustainable development in the industry.

Within 15-20 days after received payment...more