
TL-NONPVC-707 THE NONPVC film for liquid medical packaging
Material: NONPVC ROLL
Thickness 0.2+0.2mm
Size: 100MM/Customization
GRADE: pharma grade film
color: white/ customization
location: china
Usage: Pharmaceutical, dried food
- TOPLEADER
- china
- 15WORKING DAYS
- 5000T/M
- Information
- Video
- Download
The Versatile Application of Transparent NON - PVC Film
Introduction

In the modern packaging and medical industries, transparent NON - PVC film has emerged as a remarkable material with a wide range of applications. Its unique properties have made it a preferred choice over traditional materials in many scenarios. This article will explore the various aspects of transparent NON - PVC film, including its composition, manufacturing process, diverse applications, advantages, and future prospects.
Composition and Properties of Transparent NON - PVC Film
Chemical Composition
Transparent NON - PVC film is typically made from materials other than polyvinyl chloride. It often consists of polymers such as polyolefins (e.g., polyethylene and polypropylene) or other advanced synthetic materials. These materials are carefully selected to provide the desired properties without the drawbacks associated with PVC. For example, some formulations may include special additives to enhance flexibility, transparency, or barrier properties.
Transparency
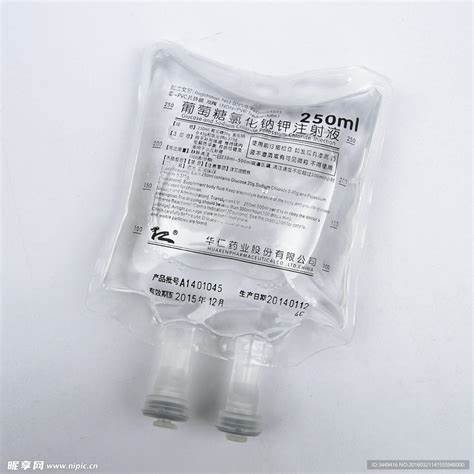
The transparency of NON - PVC film is one of its most outstanding features. It allows for clear visibility of the contents inside the packaging or medical device. In packaging applications, consumers can easily see the product, which is highly beneficial for marketing and product inspection. In medical applications, healthcare providers can monitor the contents of infusion bags or other medical containers without having to open them. The transparency is achieved through precise control of the polymer structure and processing conditions. The absence of impurities and proper alignment of the polymer chains contribute to its high - clarity appearance.
Mechanical Properties
NON - PVC film has excellent mechanical strength and flexibility. It can withstand the rigors of handling, transportation, and storage. The tensile strength of the film ensures that it does not easily tear or break under normal conditions. This is crucial in both packaging and medical applications. For instance, in packaging, it can protect the product during shipping, and in medical applications, it can maintain the integrity of medical devices during use. The flexibility of the film also allows it to conform to different shapes and sizes, making it adaptable to various packaging and medical equipment designs.
Barrier Properties
These films possess remarkable barrier properties against gases, moisture, and other substances. In packaging, this helps to preserve the quality of the product by preventing the ingress of oxygen, which can cause oxidation and spoilage, and moisture, which can lead to mold growth or product degradation. In medical applications, the barrier properties are vital for maintaining the sterility and stability of the contents within medical containers. For example, in the case of intravenous (IV) solutions, the film prevents contamination from the external environment.
Manufacturing Process of Transparent NON - PVC Film
Polymer Selection and Preparation
The first step in manufacturing NON - PVC film is the careful selection of polymers. As mentioned earlier, materials like polyethylene or polypropylene are commonly used. These polymers are sourced from reliable suppliers and undergo quality checks to ensure their purity. Once selected, the polymers may be blended with other additives such as plasticizers (if required), stabilizers, and antioxidants. The blending process is carried out in specialized mixing equipment to achieve a homogeneous mixture.
Extrusion Process
After the polymer blend is prepared, it is fed into an extruder. The extruder heats the polymer mixture to a molten state under controlled temperature and pressure conditions. The molten polymer is then forced through a die to form a continuous sheet of film. The design of the die determines the thickness and width of the film. During extrusion, the temperature profile along the extruder barrel and the die is carefully adjusted to ensure uniform melting and flow of the polymer. This results in a film with consistent thickness and quality.
Orientation and Annealing

To enhance the mechanical and physical properties of the film, it may undergo orientation processes. This can involve stretching the film in one or both directions (uniaxial or biaxial orientation). Orientation aligns the polymer chains, improving the strength and barrier properties of the film. After orientation, the film may be annealed. Annealing involves heating the film to a specific temperature and holding it for a certain period to relieve internal stresses and further stabilize the polymer structure. This step is crucial for ensuring the long - term performance of the film.
Coating and Finishing
In some cases, the NON - PVC film may be coated with additional layers to enhance its properties. For example, a coating may be applied to improve the barrier properties further or to provide a printable surface for packaging applications. The coating process can be done using various techniques such as solvent - based coating or extrusion coating. Once the coating is applied, the film may undergo finishing operations such as trimming to remove any irregular edges and winding onto rolls for storage and further processing.
Applications of Transparent NON - PVC Film in Packaging
Food Packaging
In the food industry, transparent NON - PVC film is widely used. It is used for packaging fresh produce such as fruits and vegetables. The transparency allows consumers to see the quality and freshness of the food. The film's barrier properties keep the food fresh by preventing moisture loss and oxygen ingress. For example, strawberries packaged in NON - PVC film retain their juiciness and color for a longer period. It is also used for packaging processed foods like snacks and baked goods. The flexibility of the film enables it to conform to the shape of the food products...
请用英语写一篇关于 透明 NON-PVC film 在药袋 方面的使用文章 要生动真实具体!5000字左右!谢谢
The Application of Transparent NON - PVC Film in Pharmaceutical Bags
Introduction
In the pharmaceutical industry, the choice of packaging material is of critical importance. Transparent NON - PVC film has emerged as a revolutionary option for pharmaceutical bags, offering a plethora of advantages over traditional materials. This article delves into the various aspects of using transparent NON - PVC film in pharmaceutical bags, including its material properties, manufacturing process, benefits in pharmaceutical applications, regulatory compliance, and future prospects.
Material Properties of Transparent NON - PVC Film
Chemical Composition and Structure
Transparent NON - PVC film is typically composed of materials that are free from the potential hazards associated with polyvinyl chloride. It often consists of advanced polymer blends, such as various types of polyolefins. These polymers have a well - defined molecular structure that imparts unique characteristics. For example, polyethylene - based NON - PVC films have long - chain hydrocarbon backbones, which give them excellent flexibility and stability. The absence of chlorine in the structure eliminates concerns related to the release of harmful by - products during storage or use.
Transparency

The transparency of NON - PVC film is a key feature in pharmaceutical applications. It allows healthcare providers to easily visualize the contents of the bag, which is crucial for accurate identification and inspection. The high level of transparency is achieved through meticulous control of the polymer processing. During manufacturing, the polymer chains are carefully aligned, and any impurities that could cloud the film are removed. This clear visibility enables quick assessment of the drug solution's appearance, color, and any potential particulate matter, ensuring the safety and quality of the pharmaceutical product.
Mechanical Properties
NON - PVC film exhibits remarkable mechanical strength. It has sufficient tensile strength to withstand the rigors of handling, transportation, and storage within the pharmaceutical supply chain. This strength is essential to prevent bag rupture, which could lead to drug loss or contamination. Moreover, the film's flexibility allows it to adapt to different storage and handling conditions. It can be easily bent or folded without cracking, making it convenient for packaging in various shapes and sizes of pharmaceutical bags. This flexibility also contributes to the bag's ability to absorb any external shocks or pressures during transit.
Barrier Properties
In pharmaceutical packaging, barrier properties are of utmost significance. NON - PVC film provides an effective barrier against moisture, oxygen, and other environmental factors. The film's structure restricts the ingress of moisture, which could cause hydrolysis of the drug or affect its stability. Similarly, by limiting oxygen penetration, it prevents oxidation of the pharmaceutical ingredients, thereby maintaining their efficacy. For some light - sensitive drugs, certain NON - PVC film formulations can also offer a degree of protection against UV light, further safeguarding the drug's integrity.
Compatibility with Pharmaceutical Substances
Another vital property of transparent NON - PVC film is its compatibility with a wide range of pharmaceutical substances. Unlike some materials that may interact with drugs, causing chemical reactions or leaching of harmful components, NON - PVC film remains inert. This ensures that the drug's chemical composition, potency, and purity are preserved throughout its shelf life. Compatibility testing is an integral part of the development process for pharmaceutical bags made from NON - PVC film, and extensive research is conducted to guarantee that the film does not adversely affect the drug in any way.
Manufacturing Process of Transparent NON - PVC Film for Pharmaceutical Bags
Polymer Selection and Preparation
The manufacturing process begins with the careful selection of polymers suitable for pharmaceutical applications. High - purity grades of polyolefins or other approved polymers are sourced from reliable suppliers. These polymers are then subjected to rigorous quality control checks to ensure they meet the strict requirements of the pharmaceutical industry. Before processing, the polymers may be blended with specific additives, such as stabilizers to enhance thermal stability and antioxidants to prevent oxidation during the manufacturing process.
Extrusion Process

Once the polymer blend is prepared, it is fed into an extruder. The extruder heats the polymer mixture to a molten state under precisely controlled temperature and pressure conditions. The temperature profile within the extruder is carefully calibrated to ensure uniform melting of the polymer. The molten polymer is then forced through a specialized die to form a continuous sheet of film. The design of the die determines the thickness and width of the film, which are crucial parameters in pharmaceutical bag production. The extrusion speed and other process parameters are constantly monitored and adjusted to maintain the desired film quality.
Orientation and Annealing
To enhance the mechanical and barrier properties of the film, it often undergoes orientation processes. Uniaxial or biaxial orientation can be employed, where the film is stretched in one or both directions. This stretching aligns the polymer chains, increasing the film's strength and improving its barrier performance. After orientation, the film may be annealed. Annealing involves heating the film to a specific temperature and holding it for a set period to relieve internal stresses generated during the orientation process. This step helps to stabilize the film's structure and further improve its long - term performance.
Printing and Coating (if required)

In some cases, the NON - PVC film may need to be printed with important information such as the drug name, dosage, lot number, and expiration date. Specialized printing techniques are used to ensure clear and durable printing on the film's surface. Additionally, a coating may be applied to further enhance the film's barrier properties or to provide a better surface for printing. The coating process is carefully controlled to ensure uniformity and adherence to the film without affecting its transparency or other essential properties.
Conversion into Pharmaceutical Bags
After the film is produced and processed, it is cut and sealed to form pharmaceutical bags. Advanced manufacturing techniques are used to create bags of different sizes and designs, depending on the specific requirements of the pharmaceutical product. The sealing process is critical to ensure a hermetic seal that prevents any leakage or contamination. High - quality sealing methods, such as heat sealing or ultrasonic sealing, are employed to create strong and reliable seals.
Benefits of Transparent NON - PVC Film in Pharmaceutical Bag Applications
Patient Safety

The transparency of NON - PVC film allows healthcare providers to visually inspect the drug solution before administration. This enables them to detect any visible signs of contamination, such as discoloration or particulate matter, ensuring the patient's safety. In addition, the film's inert nature and compatibility with drugs prevent any potential chemical reactions that could harm the patient. By maintaining the integrity of the drug, NON - PVC film - based bags contribute to the effectiveness of the treatment.
Ease of Use for Healthcare Providers
The flexibility and durability of NON - PVC film make pharmaceutical bags easy to handle. The bags can be easily manipulated during the preparation and administration of drugs. For example, they can be hung from IV poles without the risk of tearing, and the clear visibility allows for quick and accurate volume measurements. The user - friendly nature of these bags reduces the workload and potential errors of healthcare professionals, improving the overall efficiency of patient care.
Improved Drug Stability
The excellent barrier properties of NON - PVC film play a crucial role in maintaining the stability of pharmaceutical products. By protecting the drug from moisture, oxygen, and light, the film extends the shelf life of the drug. This is particularly important for drugs that are sensitive to environmental factors. The ability to preserve the drug's potency and quality over an extended period reduces waste and ensures that patients receive effective treatment, even if the drug has been in storage for some time.
Environmental Sustainability

Compared to PVC - based materials, NON - PVC film is often more environmentally friendly. Many NON - PVC films are recyclable or made from renewable resources. In an era of increasing environmental awareness, the use of these films in pharmaceutical packaging aligns with sustainable development goals. Pharmaceutical companies can reduce their environmental impact by choosing NON - PVC film for their bags, which can also enhance their corporate social responsibility image.
Branding and Information Display
The clear surface of NON - PVC film provides an ideal platform for branding and displaying important product information. Pharmaceutical companies can print their logos and brand names prominently on the bags, increasing brand recognition. The ability to print detailed and accurate information about the drug, including usage instructions and warnings, helps patients and healthcare providers make informed decisions. Clear and legible printing on the film also complies with regulatory requirements regarding drug labeling.
Regulatory Compliance and Quality Control
Pharmaceutical Regulations

The use of transparent NON - PVC film in pharmaceutical bags must comply with strict regulatory requirements. Regulatory authorities, such as the FDA in the United States and the EMA in Europe, have specific guidelines regarding the quality, safety, and performance of pharmaceutical packaging materials. These regulations cover aspects such as the chemical composition of the film, its physical and mechanical properties, and the absence of any leachable substances that could affect the drug. Manufacturers of NON - PVC film for pharmaceutical bags must conduct extensive testing to ensure compliance with these regulations.
Quality Control Measures

During the manufacturing process, rigorous quality control measures are implemented. Raw materials are thoroughly inspected before use, and process parameters during extrusion, orientation, and other manufacturing steps are constantly monitored. Finished films are subjected to a battery of tests, including tests for transparency, mechanical strength, barrier properties, and compatibility with drugs. Pharmaceutical bags made from the film are also inspected for proper sealing, print quality, and overall integrity. Any non - compliant products are rejected to ensure that only high - quality bags reach the market.
Standardization and Certification
To ensure the quality and reliability of NON - PVC film for pharmaceutical bags, standardization efforts are in place. International standards organizations work to establish common criteria for the material's properties and performance. Manufacturers may seek certifications such as ISO standards related to quality management systems and specific pharmaceutical packaging standards. These certifications provide assurance to pharmaceutical companies and regulatory authorities that the NON - PVC film used in bags meets the highest quality and safety requirements.
Case Studies of Transparent NON - PVC Film in Pharmaceutical Bag Applications
Intravenous (IV) Solution Bags

Many hospitals and healthcare facilities have switched to using NON - PVC film - based IV solution bags. For example, a large hospital network reported significant improvements after adopting these bags. The transparency of the bags allowed nurses to quickly and accurately assess the solution's clarity and volume, reducing the risk of errors during administration. The excellent barrier properties of the NON - PVC film maintained the stability of the IV solutions, even during extended storage periods. Additionally, the bags' flexibility and durability made them easy to handle and transport within the hospital environment.
Chemotherapy Drug Bags
In oncology treatment, chemotherapy drugs are often packaged in specialized bags. A pharmaceutical company that switched to NON - PVC film - based bags for their chemotherapy products noticed several benefits. The inert nature of the film ensured that there was no interaction between the highly potent drugs and the packaging material, maintaining the drugs' efficacy. The clear visibility of the bags allowed pharmacists to double - check the drug preparation process, enhancing patient safety. The enhanced barrier properties of the NON - PVC film also protected the light - and moisture - sensitive chemotherapy drugs during storage and transportation.
Pediatric Drug Bags

For pediatric medications, the use of transparent NON - PVC film bags has been particularly advantageous. The clear bags enable parents and healthcare providers to easily see the contents, which is especially important when dealing with small volumes of drugs for children. The user - friendly design of the bags, including their flexibility and easy - to - read labels, has improved the accuracy of drug administration in pediatric settings. The safety and stability provided by the NON - PVC film have also been crucial in ensuring the effectiveness of these delicate medications.
Challenges and Solutions in Using Transparent NON - PVC Film for Pharmaceutical Bags
Cost Considerations
One of the main challenges in using NON - PVC film is the cost. The production process of NON - PVC film, especially when meeting the high - quality requirements of the pharmaceutical industry, can be more expensive than traditional PVC - based materials. To address this, manufacturers are constantly working on optimizing the manufacturing process to reduce costs. Additionally, pharmaceutical companies can consider the long - term benefits, such as reduced waste due to improved drug stability and enhanced patient safety, which can offset the higher initial cost of NON - PVC film - based bags.
Research and Development for Specialized Applications

As the pharmaceutical industry continues to evolve, there is a need for NON - PVC film with enhanced properties for specialized drug formulations. For example, some new drugs may require even higher barrier properties or specific compatibility with novel pharmaceutical ingredients. To meet these challenges, continuous research and development efforts are focused on developing new polymer blends and manufacturing techniques. Collaboration between material scientists, pharmaceutical researchers, and packaging manufacturers is essential to drive innovation in this area.
Recycling and Disposal Management
While many NON - PVC films are recyclable, the proper recycling and disposal of pharmaceutical bags can still be a challenge. In a healthcare setting, used bags may be contaminated with drug residues, which requires special handling. Solutions include developing effective decontamination processes and establishing dedicated recycling programs for pharmaceutical - grade NON - PVC bags. Pharmaceutical companies and healthcare facilities can also work together to educate staff about the proper disposal methods to minimize environmental impact.
Conclusion
Transparent NON - PVC film has become an indispensable material in the pharmaceutical bag industry. Its unique combination of transparency, mechanical strength, barrier properties, compatibility with drugs, and environmental sustainability offers numerous benefits for pharmaceutical packaging. Despite the challenges related to cost, specialized applications, and recycling, continuous efforts in research, development, and process optimization are addressing these issues. As regulatory requirements become more stringent and the focus on patient safety and environmental protection intensifies, the use of transparent NON - PVC film in pharmaceutical bags will continue to grow. It will play a vital role in ensuring the quality, stability, and safe delivery of pharmaceutical products, thereby contributing to the overall improvement of healthcare outcomes and the sustainable development of the pharmaceutical industry.

























